Precision Oncology
Your Trusted Partner in Precision Diagnostics
Fortrea has a comprehensive understanding of the diagnostic process, from analytical validation to clinical implementation.
Strategic Regulatory Support
Knowledgeable Diagnostics Global Project Team
Extensive expertise in In Vitro Diagnostics (IVD) Development, Drug-Device Development, Companion Diagnostics (CDx), and Imaging Diagnostics
Evolution of Liquid Biopsies in Clinical Practice
Fortrea’s precision oncology experts, part of our Diagnostic Center of Excellence provide the guidance and infrastructure you need to support your liquid biopsy trials.
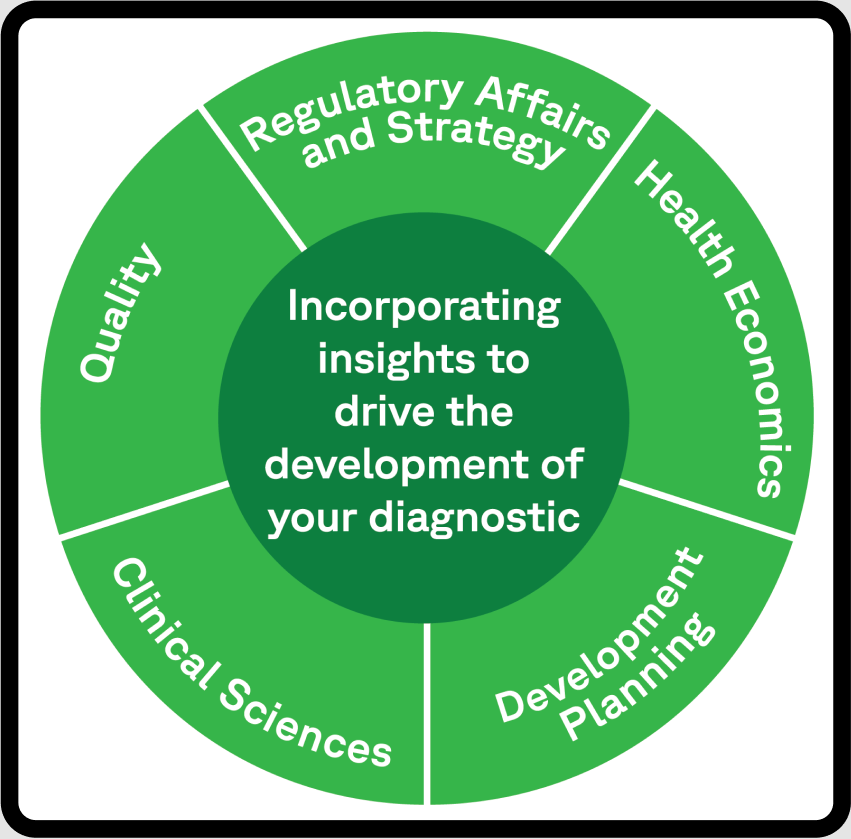
A New Approach for Diagnostic Development
Successful healthcare product development hinges on innovation to make a real impact.
At Fortrea, we use this philosophy to develop the strategies and designs needed by our stakeholders we collaborate with.
Fortrea combines specialism in.
- Regulatory strategy and regulatory affairs
- Data management and Quality Systems
- Health Economics, Outcomes and Reimbursement (HEOR)
- Tokenization
- Pre-clinical (non-clinical) testing, rational clinical trial design and conduct, ease of use, training and adoption
Oncology diagnostic data experience
24
Studies
549
Sites
95,094
Subjects
Discover the Depth of Fortrea Oncology
Explore how our oncology expertise spans the full spectrum—from biomarker-driven precision medicine to immunotherapies, targeted treatments, and advanced cell and gene therapies.

Insights