Medical Devices
Dedicated insights and know-how to advance your medical device.
Our specialized medical device division within our global CRO offers consultation and support, providing essential insights and knowledge to help you maximize your device’s innovation
End-to-end support
Enable an optimized global regulatory strategy from preclinical to your product launch and beyond.
30+ Years of Experience. One Agile Approach
Our agile, high-impact approach optimizes protocols and truncates timelines, informed by 30+ years of medical device expertise
ISO 13485 certified
Tap into the global reach and infrastructure of a full-service CRO with a global footprint in the US, EU, and APAC, an ISO 13485-certified business collaborator, and unparalleled team specialism in U.S. CFR, EU MDR, and IVDR regulations
Built Around You – More Than a Promise, It’s Our Process
We are an extension of your team, amplifying your knowledge with unmatched device-dedicated experts and a flexible, customer-centric model with optimized regulatory strategy from preclinical to your product launch and beyond, that drives seamless delivery across regions
Med device specialism—global CRO resources
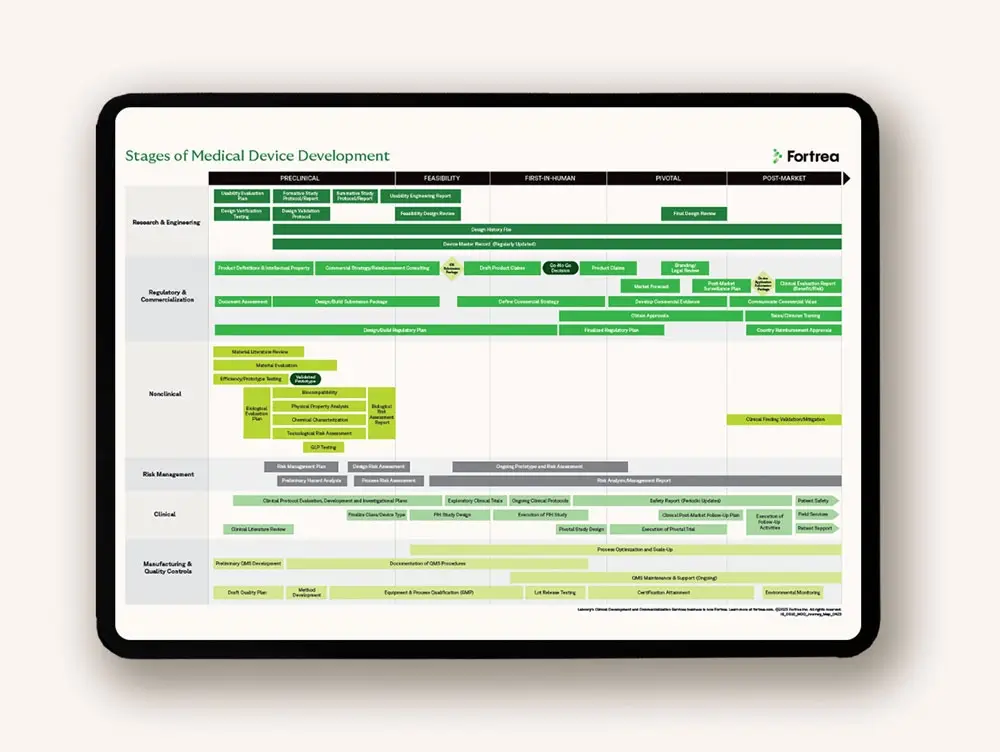
Developing medical devices requires specialized skills and knowledge of specific global regulations, protocol design, quality systems, and commercialization. Our global collaboration provides the scale, strength, and flexibility needed to advance your medical device innovation with confidence.


Drive medical device development with global regulatory expertise
Innovative technology is only the beginning. You need a solid medical device development plan that can be adapted to produce compelling evidence and a convincing value proposition. As evidence is gathered, it informs and iterates regulatory, reimbursement, clinical, and post-market strategies.
We examine every aspect of regulatory compliance to identify potential issues that could impact your design, materials, manufacturing methods, or financial plans. Leveraging worldwide experience with regulatory agencies, we develop novel, targeted strategies to help you achieve regulatory approval in your target markets.
Enable efficiency across your medical device development
Every trial has unique characteristics. Combined with optimized study execution, we will deliver the evidence you need for your submission while saving you time and money during the operational phase of your project.
Our advisors’ deep experience can help you identify ways to improve efficiency and, where possible, reduce your timelines. They also work with you to pivot your strategy if unexpected findings occur, remaining flexible to meet unique study requirements and timelines. Let us collaborate with you to accelerate medical device innovation from the beginning to improve efficiency and speed your time to market.
Medical Devices related frequently asked questions
Choose the level of oversight and accountability you need.
-
How does the FDA classify medical devices?
Medical Device classification includes three categories based on risk:
- Class I: Low risk (e.g., bandages)
- Class II: Moderate risk (e.g., infusion pumps)
- Class III: High risk (e.g., heart valves)
-
What is a 510(k) submission?
A 510(k) is a premarket submission to the FDA demonstrating that a device is substantially equivalent to a legally marketed device.
-
What is a PMA (Premarket Approval)?
PMA is the FDA process of scientific and regulatory review for Class III devices to ensure safety and effectiveness.
-
What are the responsibilities of manufacturers?
Manufacturers must ensure devices meet regulatory standards, report adverse events, and maintain quality systems.
-
What is post-market surveillance?
It involves monitoring the safety and performance of a device after it has been approved and is on the market.
21 CFR Part 812 (FDA) – In the U.S., clinical trials are regulated under this code, which governs Investigational Device Exemptions (IDEs) and ensures compliance with FDA requirements
-
What is regulatory compliance for medical devices?
- ISO 13485- Based QMS
- Risk Management
- Labeling & Documentation
- Post-Market Surveillance