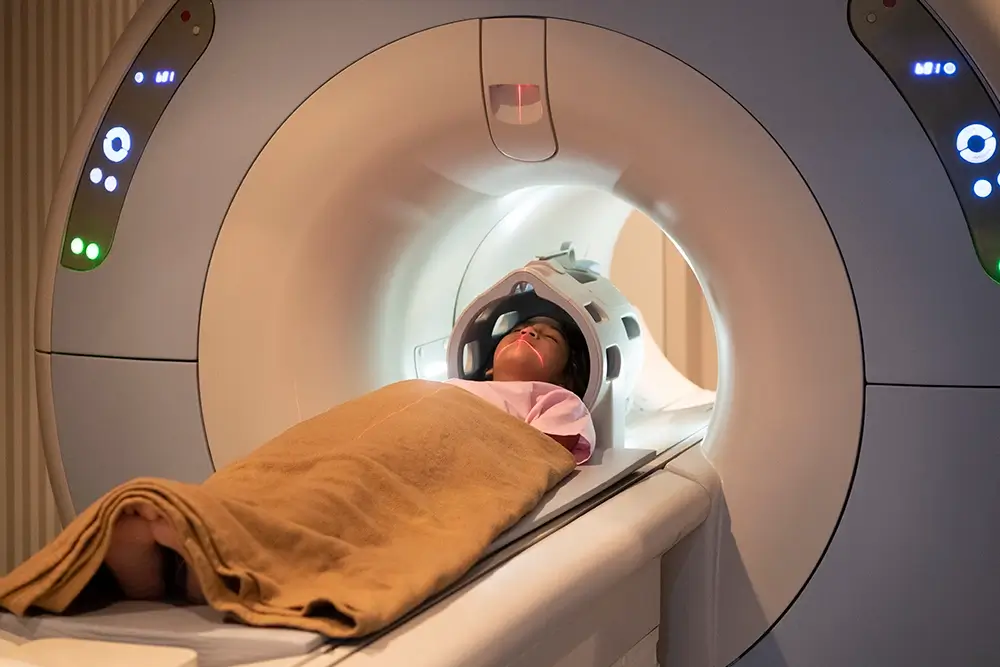
Neuroscience
Driving innovative advances in neuroscience studies
Increase the likelihood of seamless trial conduct with Fortrea. Apply a science-driven approach to navigate the challenges of neuroscience clinical trials.
Tailored technologies and solutions
Access a unique combination of science, expertise, digital technologies and data to enable the successful delivery of your neuroscience clinical trial.
A focus on patients
Apply our scientifically rigorous patient-centric strategy to reduce the participation burden and maximize the value of your study.
Seamless collaboration
Optimize your clinical research with our strategic product development methods for advancing neuroscience therapies and precision medicines.
The catalyst for your next breakthrough in neurotherapeutics
We help you manage complexity to keep your neuro development moving forward. Making gains in the dynamic landscape of neuroscience drug development requires flexibility to adapt to changing project requirements and drive efficiencies. Whether it’s establishing the right endpoints, finding the right sites or measuring complex neuro data, our industry-leading experts apply years of experience in neurodegenerative and psychiatric disorders to design and implement clinical trials that can handle the inherent complexities of product development—from early phase through post-marketing and beyond.


Effective, patient-centric strategies
We help optimize protocols and increase the likelihood of your trial’s success. Neuroscience has a broad range of disorders including neurodegenerative, developmental, psychiatric, acute critical care, as well as special patient populations like elderly, rare spectrum or pediatric that are highly sensitive to risks from age- or mental-related challenges, noncompliance and psychological instability.
We use innovative approaches to recruit and retain these individuals and we provide insightful methods for wet, tissue imaging and digital biomarker collection to support precision medicine and we combine this with our commitment to patient centricity through our real-world data and partnerships with patient advocacy groups.
The right endpoints to maximize differentiation
We help increase the chances for meeting your program objectives by working with you to select the right endpoints. We combine our established strategic partners with expertise in neuro-specific clinical outcome assessment (COA), imaging and digital technology. We then apply our multifaceted neuroscience clinical expertise to support your selection of the right neurological outcome measures, enable continuous rating surveillance to monitor the integrity of your endpoints and ultimately maximize the differentiation of your product.


Get the high-performing sites you need
We help you reduce your trial’s startup time by finding investigators with the highest chances of success through our strong partnerships with neuroscience-specialized sites, augmented by the industry’s deepest performance data. We then enhance site performance, delivering training to sites on the application of complex routes of administration, such as intrathecal or intracerebral delivery.
Behind every Alzheimer’s Disease trial is a story
Our dedication to Alzheimer’s Disease
A unified, experienced neuroscience team with over 30 years of therapeutic leadership, led by Dr. Leone Atkinson, MD, PhD. Combining depth of academic excellence with an agile approach to real-world drug development experience.
Patient centricity
Patient centricity is core to our methodology. We engage and collaborate with patients and advocacy groups to drive optimally designed protocols and design patient materials to support recruitment.
Digital Health Innovations (DHI)
We integrate approaches that minimize the impact of trial participation on Alzheimer’s Disease patients’ lives - removing barriers to participation and increasing trial access, patient recruitment, engagement and retention.
Cell and Gene therapy opportunities in Alzheimer’s Disease
Delivering complex cell and gene therapy trials for Alzheimer’s Disease requires deep experience and specialized capabilities, from the right site selection to advanced procedural training and patient and family support. Framed by Carole Stark’s personal story and hope for future generations, the video highlights how innovative neuroscience research is helping shape a future where Alzheimer’s may no longer be a one‑way journey.
The Stark’s story
Carole and Hank Stark share their emotional journey through Alzheimer’s Disease, from the fear and isolation of early symptoms to the hope they found through joining a clinical trial. Their story highlights how research offered not only support for Carole, but renewed optimism for their children and future generations.
Site-CRO relationships
Fortrea’s strong, collaborative site relationships are at the heart of successful Alzheimer Disease clinical trials. By partnering closely with investigators, incorporating real‑world insights into protocol design, and maintaining consistent communication, Fortrea ensures high‑quality delivery while supporting patients through long, demanding studies.
Neuroscience experience that matters
In the last 5 years, we have supported:
Data covers the period from 2020 through 2024.
Our top areas of neuroscience expertise
Together, exceptional research is possible.
-
Neurodegenerative disorders
- Alzheimer’s disease (AD)
- Parkinson’s disease (PD)
- Multiple sclerosis (MS)
-
Psychiatric disorders
- Autism spectrum disorder
- ADHD
- Schizophrenia
-
Pain
- Chronic
- Acute
- Migraine
- Neuropathies
-
Rare neurological disorders
- Fragile X syndrome (FXS)
- Mucopolysaccharidosis
- Muscular dystrophies
- Batten disease
-
Other neuroscience areas
- Epilepsy
- Stroke
Our experts
Delivering neuroscience leadership
An exceptional partnership starts with an experienced team. Browse the bios of our featured neuroscience team members.
Fortrea is a trusted, knowledgeable partner. The team is always willing to go above and beyond to meet study needs and deliverables. They have a good working relationship with our sites and a strong commitment to our trial and the patients on study.
Large Biotech
The team is always willing to go above and beyond
Insights